TRAINING ON INTRODUCTION OF MEDICAL DEVICE REGULATORY FRAMEWORK IN MALAYSIA

TRAINING ON INTRODUCTION OF MEDICAL DEVICE REGULATORY FRAMEWORK IN MALAYSIA
The Medical Device Authority (MDA) will be organizing a training session titled “Training on Introduction of Medical Device Regulatory Framework in Malaysia” as part of its continuous effort to enhance industry understanding of Malaysia’s medical device regulatory framework.
📅 Date: Tuesday, 25 November 2025
🕣 Time: 8.30 a.m. – 1.00 p.m.
📍 Venue: Medical Device Authority, Cyberjaya
💰 Training Fee: RM1,000 (HRD Corp Claimable)
🗓️ Registration Deadline: 12 November 2025
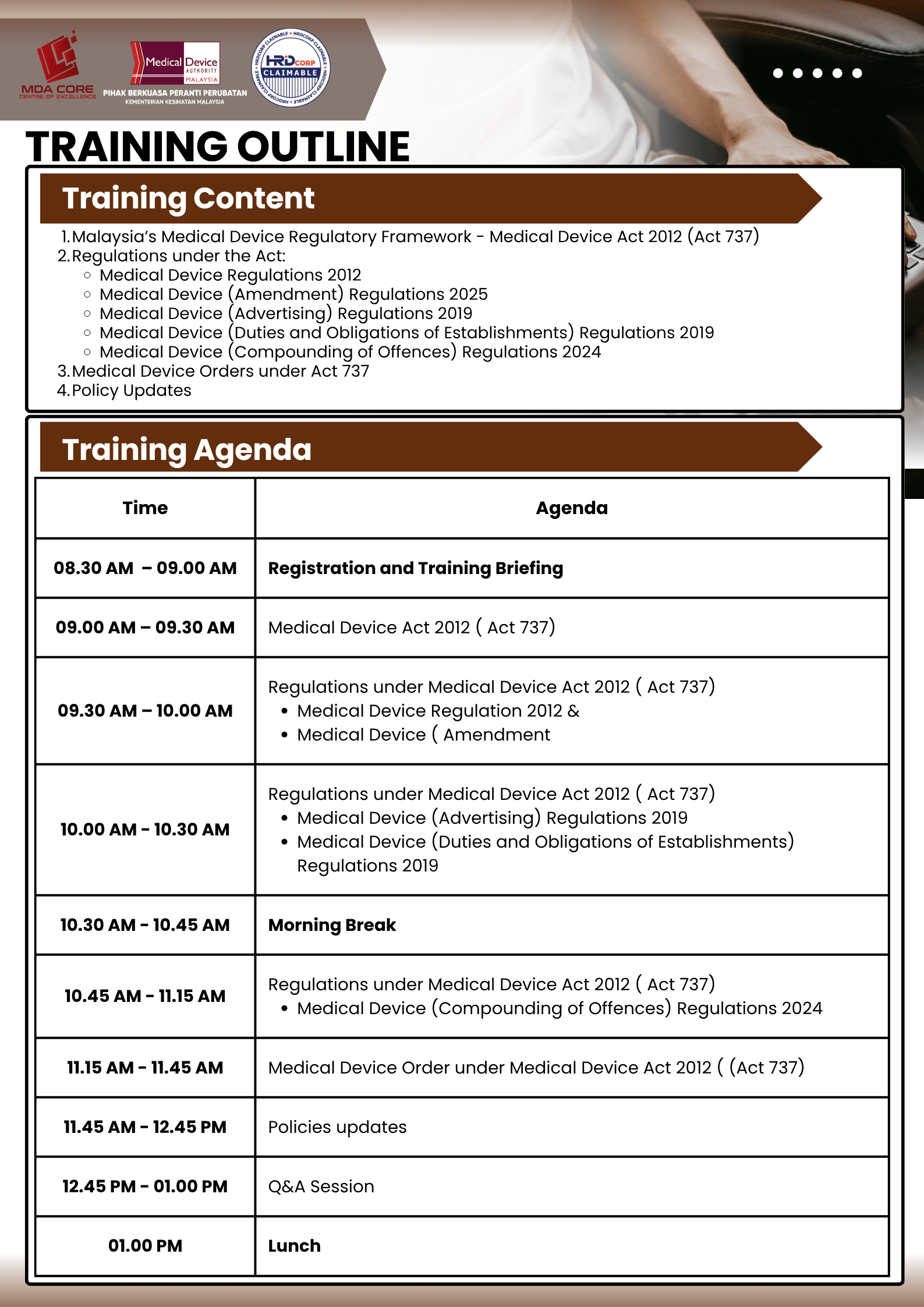
This training aims to provide participants with a comprehensive understanding of the Medical Device Act 2012 (Act 737), key regulations, subsidiary legislations, and the latest policy updates introduced by MDA.
Training Objectives:
-
Understand the structure and purpose of the Medical Device Act 2012 (Act 737).
-
Identify and describe the main regulations and subsidiary legislations under the Act.
-
Recognize the roles and responsibilities of establishments (manufacturers, importers, distributors, ARs).
-
Gain awareness of the latest amendments and policy updates related to medical device regulation.
-
Appreciate the importance of compliance in ensuring patient safety and market integrity.
Who Should Attend:
Regulatory affairs officers, quality assurance and management personnel, medical device establishment representatives, and healthcare professionals seeking regulatory understanding.
📱 Scan the QR Code in the poster to register.
Upon acceptance, an invoice and payment details will be issued within 2–3 working days.
For further information, please contact MDA CORE Training Secretariat at [email protected]
Prepared by: MDA CORE (Administrative Division)
Uploaded by: Corporate Communication Division
Date of Publish: 5/11/2025






