ADVERTISEMENT OF MEDICAL DEVICE
What is an Advertisement?
 Legal Framework
Legal Framework
Section 44 (1): No person shall advertise a medical device unless the medical device is registered and complies with the Act.
Section 44 (2): Advertisements must not make any misleading or fraudulent claims.
Medical Device (Advertising) Regulations 2019
No person shall advertise a registered medical device without approval from the Authority.
How to Submit a Medical Device Advertisement Application?
To submit an advertisement application for medical devices, please refer to the following guidelines and resources that clarify the requirements for advertisements that require or do not require approval:
Reference Documents:
- Guidance Documents MDA/GD/0032: Code of Advertisement – Outlines the standards and principles for advertising medical devices.
- Guidelines: Application for Medical Device Advertisement Approval - Requirements – Details the approval process and necessary documentation.
Application Form:
- Advertisement Application Online Form – Digital submission platform for advertisement approval.
- Template for an Advertisement Letter of Authorisation – Standard template for authorisation letters related to advertisement applications.
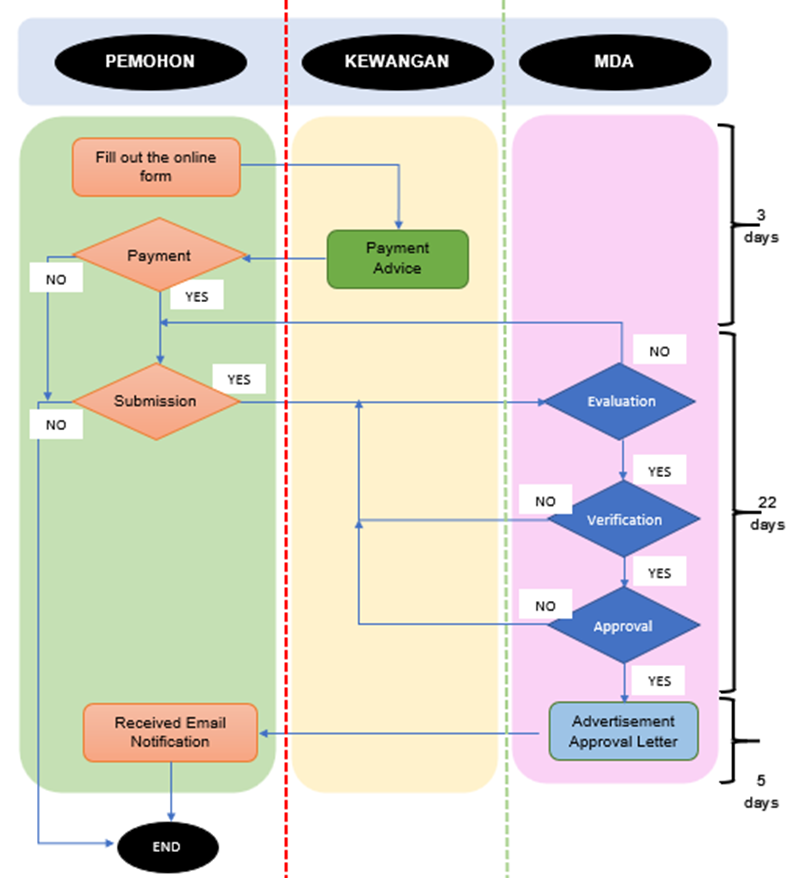
The process flow for an advertisement application is as follows:
|
IMPORTANCE NOTICE: 1. Claims Must Be Accurate and Aligned with Registration All advertising claims must strictly align with the product’s approved registration. Claims must be consistent with the intended use, fully supported by documentation. Any advertisement containing exaggerated, unsupported, or unapproved claims will be rejected. 2. Prohibited Claims and Content All advertisement claims must be developed in accordance with the Code of Advertisement (COA). |
Contact Information
Advertisement Application Process (e.g. application submission) :
- General Email: [[email protected]]
- Administration : Mohamad Aznil | Tel: + (03-82300 0247)
Officers in Charge :
- Hasdiana Mohammadiah | ☎ +603-8230 0364 | 📧 [email protected]
- Nur Maizura Zarmani | ☎ +603-8230 0339 | 📧 [email protected]
- Dinesh Murgis | ☎ +603-8230 0393 | 📧 [email protected]
- Zahroh Hasanah Darwis | ☎ +603-8230 0388
Prepared by: Advertisement Unit ( Pre Market Control Division)
Uploaded by: Corporate Communication Division
Date of upload: 5 February 2026






