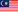REGISTRATION NOW OPEN – DON’T MISS OUT! TRAINING ON COMPLIANCE ON GDPMD REQUIREMENTS (SESSION 2)
Greetings from the Medical Device Authority (MDA)!
We are pleased to announce that MDA will be conducting Training on Compliance on GDPMD Requirements – Session 2.
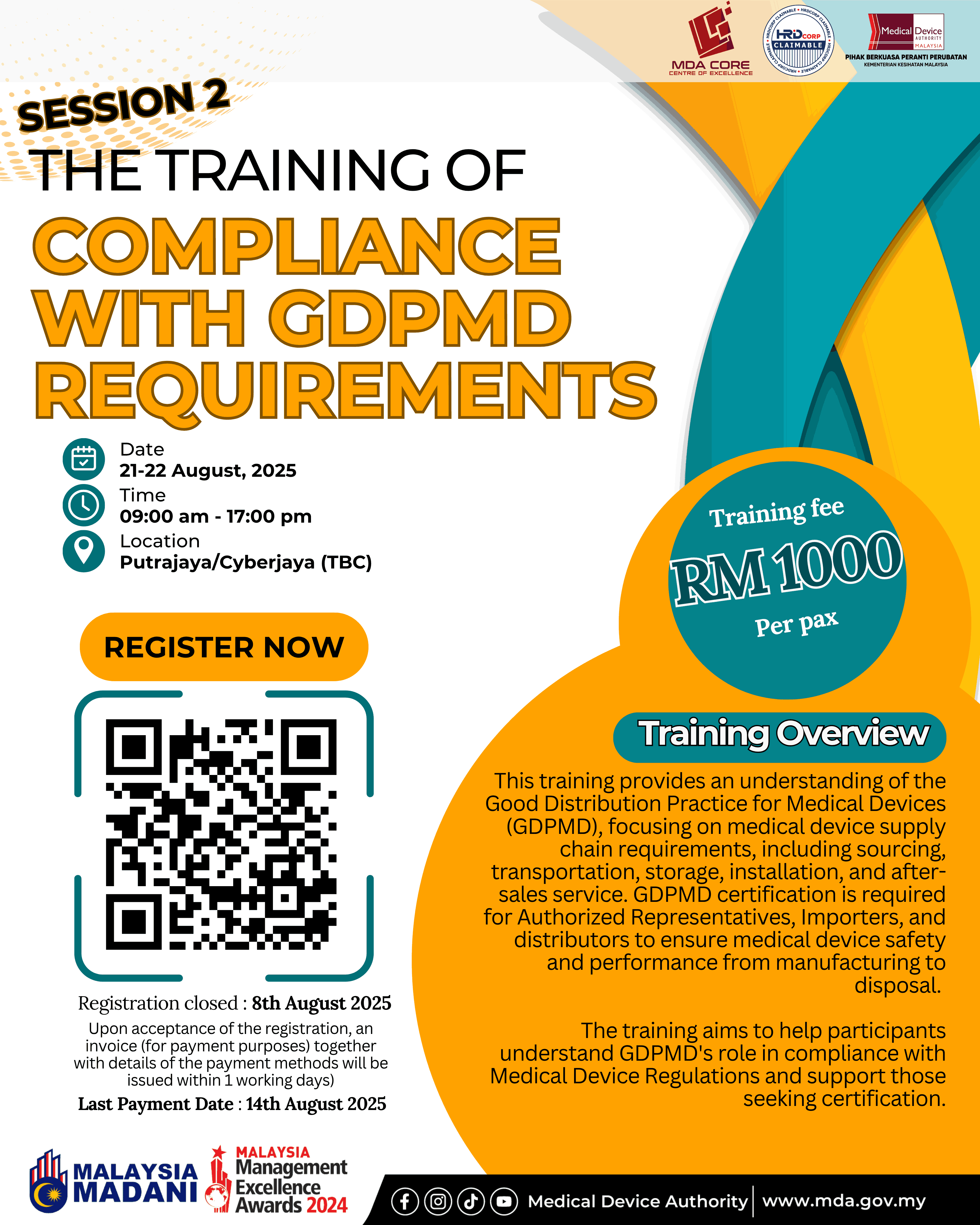
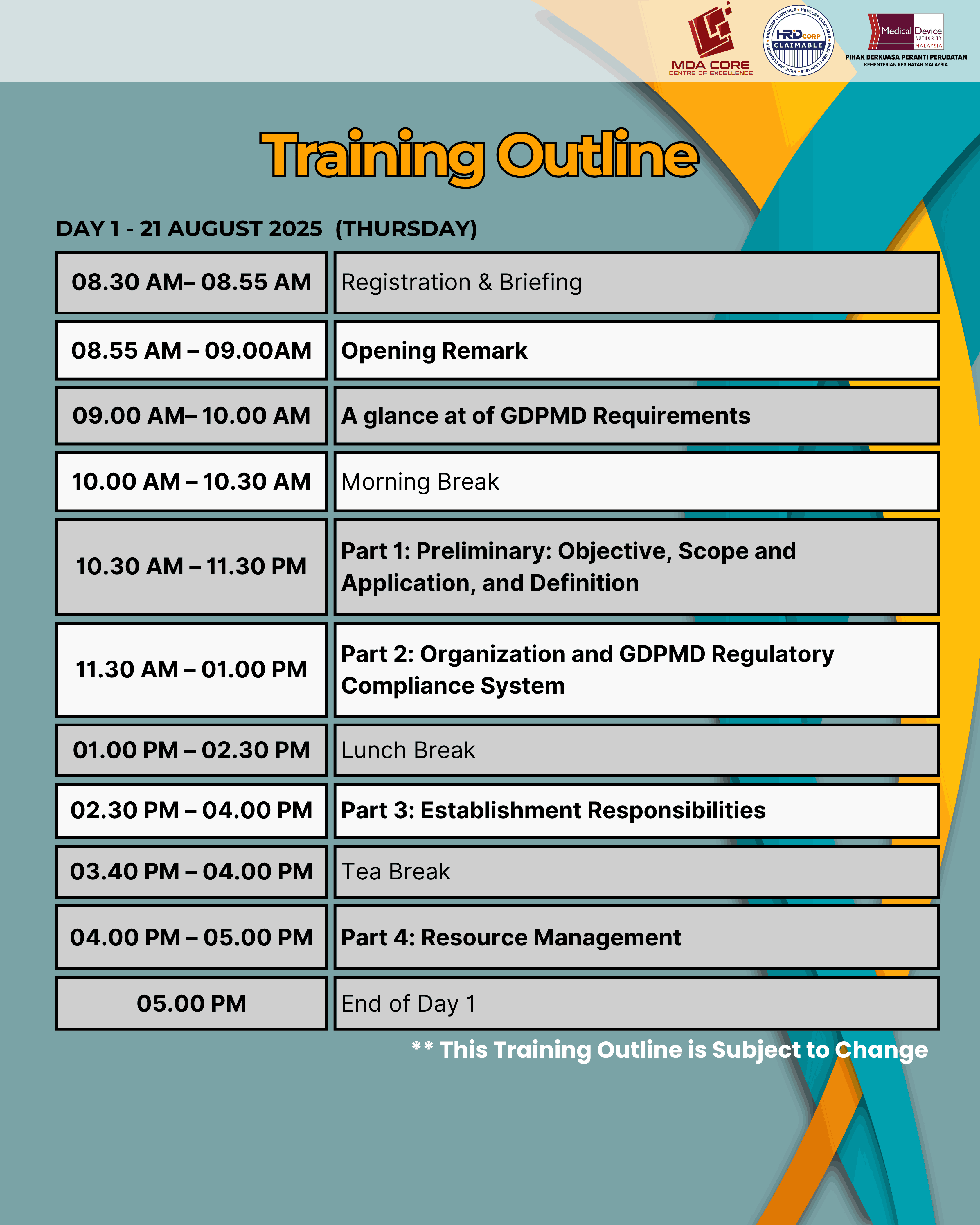
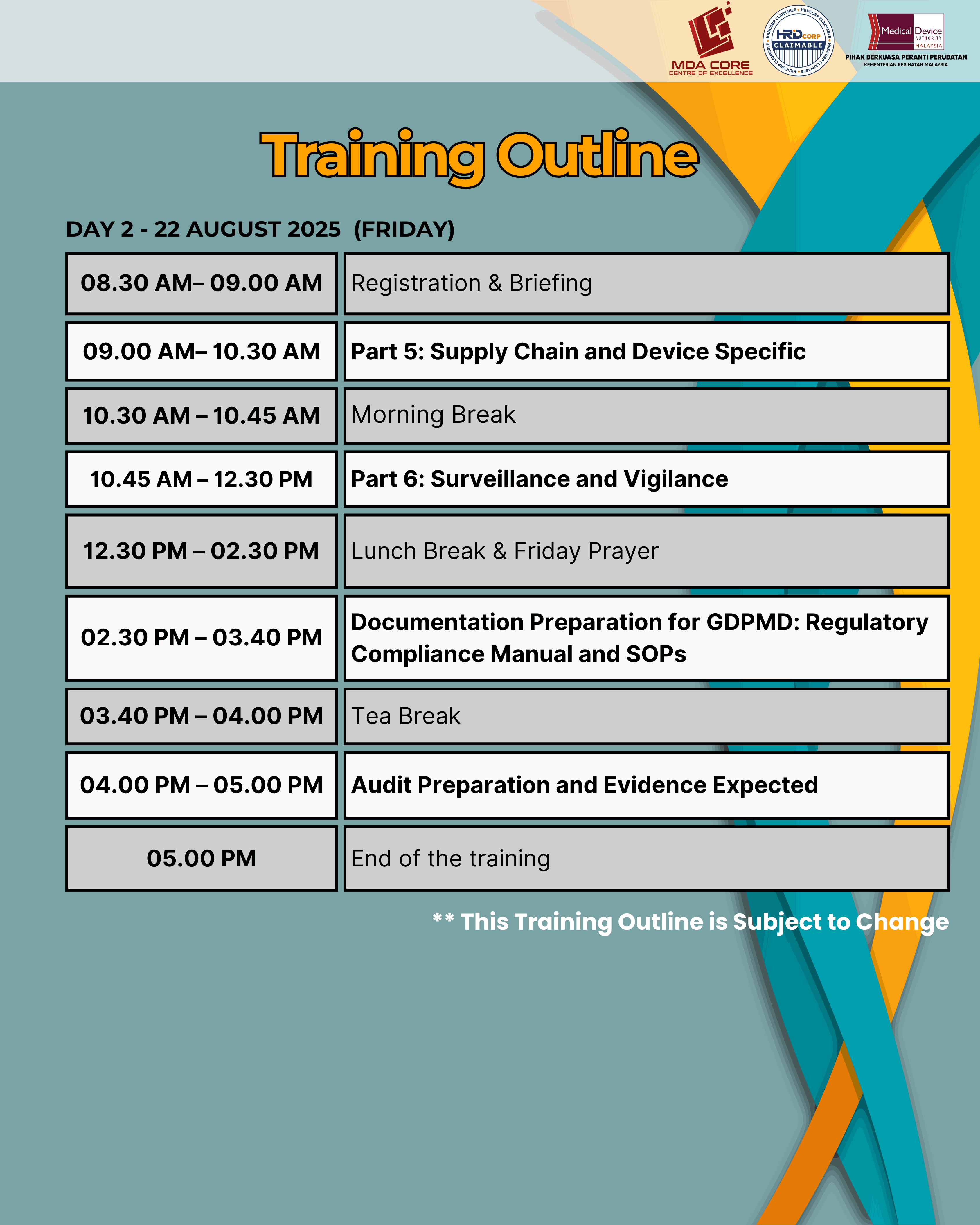
This training is to provide an understanding of the MDA/RR No. 1, Good Distribution Practice for Medical Devices (GDPMD), addressing requirements in the medical device supply chain, which include, but are not limited to, product sourcing and procurement; transportation and delivery; storage; installation, commissioning, service and maintenance, calibration and after‑sales service; tracking, documentation and record‑keeping practices.
In order to carry out activities relating to medical devices, certification to GDPMD is required for establishments such as Authorized Representatives, Importers, and Distributors in applying for establishment license. This is to ensure the safety and performance of medical devices are preserved post‑manufacturing until their disposal.
This Session 2 training aims to help participants in understanding the scope and use of GDPMD in complying with the Medical Device Regulations and to assist participants intending to pursue GDPMD certification.
- Registration is now open until 8th August 2025.
Register here: https://forms.gle/QsK9xiDV8mwk2Fbd6 or scan the QR code from the poster.
- This training is claimable under the HRDCorp SBL Scheme, subject to HRDCorp’s terms and conditions.
For further inquiries, feel free to reach out to us at [email protected].
We look forward to your participation in Session 2 of this important training!
Thank you.
Prepared by: Administrative Division (MDA CoRE)
Uploaded by: Corporate Communication Division
Updated date : 23rd July 2025