MDA INFORMATIVE SESSION II
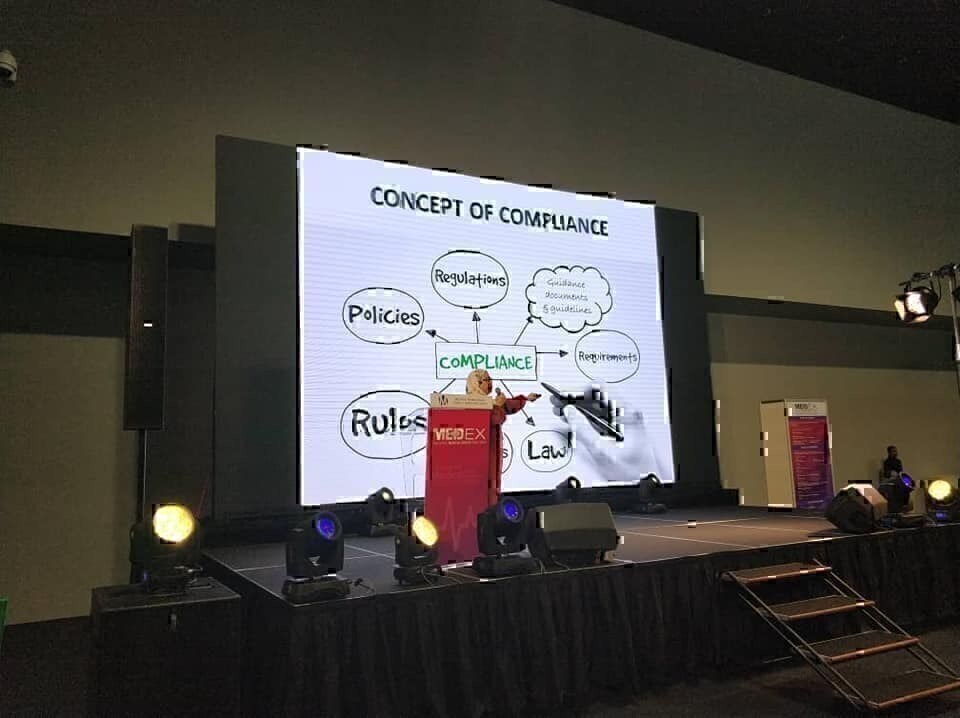
KUALA LUMPUR, 24 October 2018 - MDA Informative Session II was held at hall 5, level 2, MITEC, KL, in conjunction with MyMEDEX 2018 and 23rd AHWP Meeting. This session highlighted updates on the implementation and enforcement of Medical Device Act 2018 (Act 737) and was attended by around 100 participants from the medical devices industry.